Pharmaceutical Analysis LABORATORY
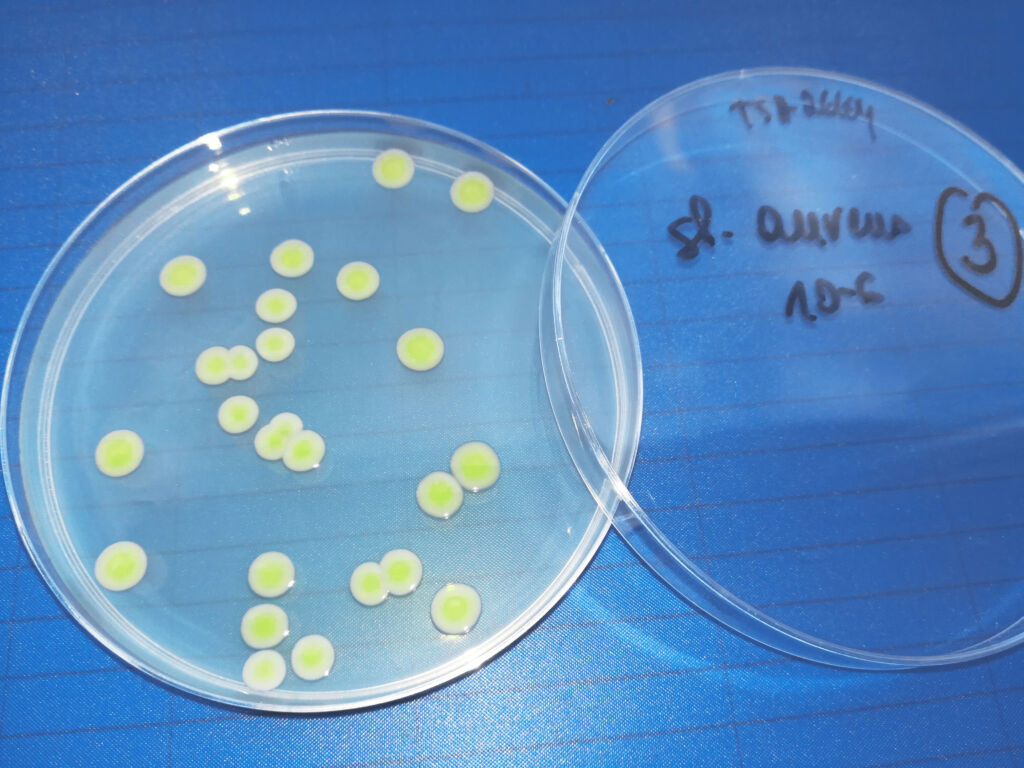
Microbiological Analysis Section
We offer comprehensive support in microbiological testing for pharmaceutical companies. Thanks to our experience, GMP certification and regular audits, we provide our clients with reliable, safe and compliant test results.
In addition to standard quality control testing, we offer the development of dedicated microbiological methods, precisely tailored to the specific characteristics of pharmaceutical products and non-medicinal products. This provides our customers with reliable, safe and fully compliant solutions that enhance the quality and credibility of their products.
SCOPE OF ACTIVITIES:
- sterility testing
- microbiological purity testing (including testing of controlled substances Gr I – N)
- testing of bacterial endotoxins (gel method: limit test)
- antimicrobial efficacy testing
- effectiveness of disinfectants
- microbiological monitoring of clean rooms
- testing of the fertility and sterility of microbiological media
- maintaining a collection of pharmacopoeial and environmental strains
- validation and transfer of microbiological methods – confirmation of the suitability of a method for a given application.
- filtration pumps (Steritest SYMBIO PUMP)
- air sampler, e.g. MAS 100 NT
- water bath with digital temperature control, dryer, refrigerators, freezers, scales
- incubation equipment – incubators that operate within a specific temperature range of 20–25, 30–35, 35–37, 42–44,
- chambers with laminar air flow, known as LAF